About 21 million Americans suffer from major depressive disorder (MDD), according to data from the
The FDA approved the use of
The earlier you start extreme depression treatment, the higher the chances you avoid serious consequences of this condition. Book an appointment today!
How Does Pristiq Work?
Pristiq belongs to a class of medications called serotonin-norepinephrine reuptake inhibitors (SNRIs). This group of drugs works by increasing the levels of two neurotransmitters (brain chemicals), preventing their reabsorption in the brain. As such, the increased levels of the two neurotransmitters (serotonin and norepinephrine) aid in better communication between neurons resulting in better moods and lowering depression symptoms.
What is Pristiq Used for?
The FDA currently approves Pristiq medication for depression only. However, there are other off-label uses for Pristiq. Off-label uses are those for which a drug has not been approved but has nonetheless shown effectiveness in treating. Pristiq off-label uses include:
- Managing menopausal hot flashes. Pristiq has shown
effectiveness in reducing the number of hot flashes and their severity[3] . - Treating anxiety and social anxiety disorder. Pristiq has shown potential to be effective in treating anxiety and social anxiety disorder, though in
small studies[4] and inanother review[5] , it showed effectiveness in treating anxiety as a symptom of depression. - Diabetic neuropathy. A
study[6] has shown that Pristiq is more effective in treating pain resulting from diabetic neuropathy than a placebo. Diabetic neuropathy is a condition where patients suffer nerve damage from diabetes.
Consult a doctor to get a diagnosis and the most suitable medication prescribed.
Pristiq Dosage for Depression
Pristiq is available as an oral extended-release tablet. It comes in three strengths: 25 mg, 50 mg, and 100 mg. It is taken once daily since the contents of the tablet release slowly throughout the day as an extended-release drug.
Pristiq dosage is dependent on several factors, including a patient’s general health, the severity of their condition, and any other medication they could be taking. The dosage for depression ranges from 50 mg to 400 mg. The most common dosage is, however, 50 mg a day. The doctor will adjust the medication according to a patient’s response.
The maximum Pristiq dosage when treating depression is 400 mg a day. But for patients having liver or kidney disease, it is lower.
Patients can take Pristiq with or without food. But they should not chew, crush, or dissolve the drug in water as that could release all the drug content at once, increasing the risk of severe side effects.
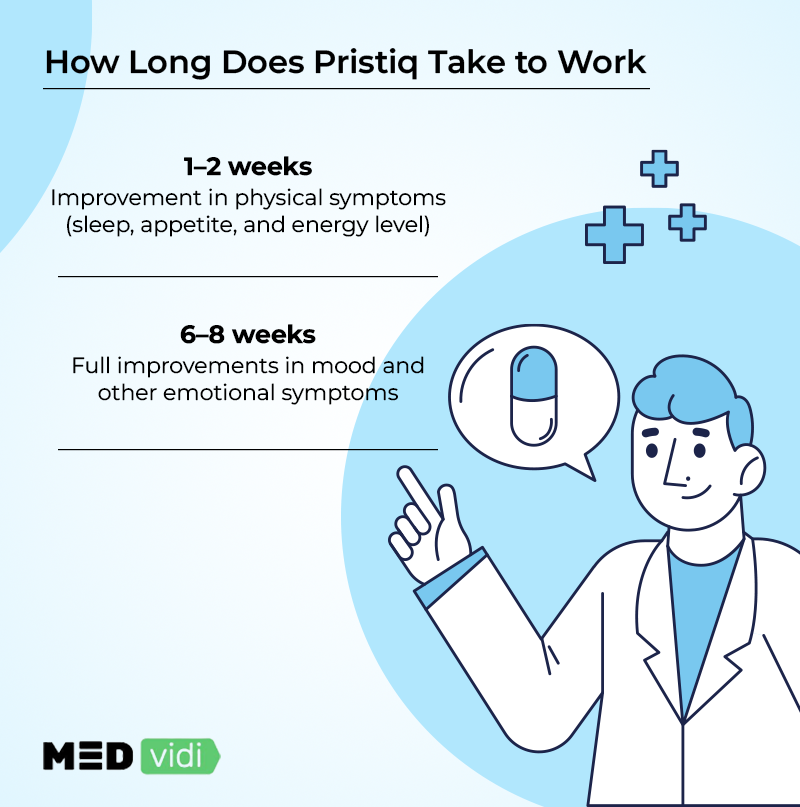
How Long Does Pristiq Take to Work?
Pristiq, like other SNRIs, takes time to build up in the body, and the brain chemicals will also require time to get to sufficient amounts to result in positive changes. It can therefore take about 2-3 weeks to improve physical symptoms, enhancing sleep, appetite, and energy. It can, however, take about 6-8 weeks for full improvements in mood and other emotional signs.
Follow the doctor’s instructions precisely, including the dosage and intake time, and tell about any side effects or inefficacy of the meds you notice.
Pristiq Side Effects
Patients may experience some side effects, but most are usually mild, and severe ones are often rare.
Common side effects include:
- Headache
- Dry mouth
- Vomiting
- Fatigue
- Diarrhea
- Insomnia
- Restlessness
- Constipation
- Loss of appetite
- Vomiting
- Feeling nervous
- Nausea
- Increased sweating
- Tremors
- Sexual problems
Severe side effects include:
- Suicidal thoughts in young patients
- Seizures
- Low sodium levels
- Increase in blood pressure
- Serotonin syndrome
Pristiq Withdrawal
Pristiq has the potential to cause discontinuation syndrome. This condition happens because the body gets used to the drug’s effects, so stopping it suddenly shocks the body and requires time to adapt to functioning without the drug. Patients should not stop taking Pristiq without their doctor’s advice, or they can experience withdrawal symptoms:
- Increased irritability
- Trouble sleeping
- Headache
- Nausea
- Diarrhea
- Confusion
- Seizures
If there is any reason for patients to stop taking the drug, the doctor will guide them in tapering off the dosages until they only take the 25 mg tablet daily, allowing the body to adjust.
Conclusion
Pristiq antidepressant is an effective medication for adult patients with depression. It is also one of the few antidepressants that doctors can prescribe for long-term use, as there is minimal risk of long-term side effects. However, doctors recommend that patients give the drug time to determine whether it is working or not. Often different people may have varied reactions to it. Above all, patients should follow the doctor’s directions, including when to stop using the drug.













